Overview
CBE GMP Uplift Programs will provide participants with a real-world perspective on Good Manufacturing Practice (GMP) and are designed to assist with the interpretation and application of GMP into practice, for those involved in life science products. CBE brings strong technical credentials, with experience across large and small Biologics, Pharmaceuticals, Regenerative Medicine and Animal Health companies, both in consulting and GMP related enterprise training.
Through a competitive process, MTPConnect's Researcher Exchange and Development within Industry (REDI) initiative funded by the Medical Research Future Fund (MRFF) selected CBE to develop and deliver a new program addressing key skills gaps in the sector workforce (REDI Impact Report). MTPConnect partnered with experienced organisations to deploy an integrated three-pillar plan driving skills and workforce development for the medical technology, biotechnology and pharmaceutical (MTP) sector nationally.
CBE lead's the Consortium, accompanied by ARCS Australia and Merck Life Sciences Australia; CBE Pure Solutions (VIC), Translational Research Institute TRI (QLD) and UTS Biologics Innovation Facility (BIF) (NSW) facilities are used for hands-on training in a GMP-like environment. The Programs feature high calibre, relevant Guest Lecturer faculty to share real-world experiences.
CBE’s GMP Uplift Programs will provide participants with an industry relevant, practical and experiential GMP learning experience.

Relevant
Practical
Experiential
At a glance
Essential GMP Program
The Essential GMP Program is designed for industry, entry level employees (new starts up to 12 months) and is offered as a 2-day equivalent online only or a 5-day equivalent course, combining online, electives and face-to-face workshops, all designed for participant flexibility and to upskill in core GMP principles. The courses include:
The Essentials GMP Online Program, builds the theoretical concepts of a Core GMP framework for all students, covering the GMP basics.
- 6 Core modules covering fundamental concepts of GMP delivered virtual online.
The full Essentials GMP Program, builds the theoretical concepts of a Core GMP framework, combined with the application of theory into practice.
- 6 Core modules covering fundamental concepts of GMP delivered virtual online.
- Complementing this, participants will choose 2 Elective modules across Biologics, Pharmaceuticals, Regenerative Medicines and Quality Control delivered virtual, with facilitated online webinars for Q&A.
- The face to face workshop days days are onsite and cover a GMP Workshop of case studies and a GMP Hands-on Workshop in an experiential GMP environment.
- These are offered on scheduled days in a GMP-like facility either CBE Pure Solutions or Jumar Bioincubator (VIC), TRI T3 Cleanrooms (QLD) or UTS Biologics Innovation Facility (BIF) (NSW).
Advanced GMP Program
The Advanced GMP Program, is for those working in a GMP role (12 months+) looking for an in depth understanding of the critical areas that underpin GMP compliance in practice. It includes:
- 6 Advanced short courses, are complementary and for those wanting to upskill.
- Each course will utilise a theory session, case study to demonstrate theory in practice and a newly designed facilitated workshops exploring the case study learnings.
- Designed for busy professionals, the modules will have online components, with virtual facilitated sessions.
All CBE programs will have real world examples, case study work, resources and tools, guest lecturer content and assessments for certification.
Click here to register for a place in the GMP Uplift Program or log in to the GMP Uplift LMS
GMP Uplift Consortia
The COVID-19 pandemic has brought unprecedented focus on the Medical Technology, Biotechnology and Pharmaceutical (MTP) sector; highlighting the importance of research and development, new technologies and advancements to navigate human health challenges. This coupled, with the importance of sovereign capability and a robust supply chain to support the provision of essential MTP products. This has created unique opportunities to invigorate and invest for a stronger sector. However, these investments require the supportive infrastructure to ensure they can be realised. Skilled labour, in particular skilled in the manufacture of these MTP products, namely competency in GMP, is a vital piece of the puzzle.

The GMP Uplift training programs are developed by the Centre for Biopharmaceutical Excellence (CBE). CBE brings strong technical credentials, with experience across large and small Biologics, Pharmaceuticals and Regenerative Medicine companies, both in consulting and GMP related enterprise training. This coupled with extensive experience in GMP operations, compliance, auditing and QMS design, a wide industry network for guest lecturers and readily available case studies.
The GMP Uplift Consortia bring an experienced team, industry relevant content, state-of-the-art facilities and trainers to deliver programs to uplift GMP related skills. The team are committed to providing relevant, practical and experiential GMP education to develop the next generation of advanced manufacturing workforce to support Australia’s MTP sector and its growth plans.

ARCS Australia has a strong connection with large Pharma and MedTech companies operating in Australia, extensive experience in developing, organising, hosting and administering educational training content. ARCS has LMS and virtual Community engagement infrastructure and a talented team to manage the course administration and reporting elements to complement CBE’s technical experience. It also brings a wealth of experience in education and training within the MTP sector.

Merck Life Sciences Australia brings to the consortium significant hands-on experience in quality control methodology, particularly around biologics and microbiology technology. Merck regularly training their customer base in the application of their equipment and methods and will be able to lead the quality control hands on component of the course.

CBE Pure Solutions brings to the consortium an advanced specialist hands-on facility specifically designed for teaching in Melbourne VIC.

TRI T3 Cleanrooms bring to the consortium a state-of-the-art facility set up for biologics manufacture, in Brisbane QLD.
UTS Biologics Innovation Facility (BIF) brings to the consortium a state-of-the-art dedicated GMP-ready biologics facility set up for pilot scale manufacture, in Sydney NSW.
The facilities are used to train people in applications relevant to GMP, such as gowning, cleanroom behaviour, operating in a GMP environment, environmental monitoring, microbiological techniques and aseptic practices.
Essential GMP Uplift
Training for Entry Level Employees
CBE consultants have worked in industry and consulted for decades within evolving GMP environments. This background has enabled us to have a clear vision of what is needed for entry level employees. We understand that, without context, the GMP rule can be confusing and seem somewhat arbitrary. This is the reason for the Core GMP modules, which are technology independent and provide fundamentals of why, what and how GMP rules are applied in different work functions and cover universal requirements/GMP principles. The Core GMP Modules will be online, have an assessment and are a pre-requisite for the Electives and Workshops. Prior to entry into the course participants will be required to pre-read resource information.
The four Electives are more specific GMPs tailored to each of three technologies plus laboratory Quality Control and provide GMP expectations within different technologies. Participants complete two Electives, allowing them to stream to technology GMP modules that that relate to their workplace and have more specific examples, case studies and guest lecturers. The Electives will be virtual, on-line, facilitator lead and have an assessment.
On the completion of the six Core and two Elective GMP Modules, students will do the two workshops are designed for students to apply their GMP learnings. A one-day, face-to-face facilitator led, onsite Case GMP Workshop for feedback, problem sharing, group practice using case work, an opportunity to engage in Q&A and networking.
The last day enables the participants to apply in practice within a facility in a “hands on” learning environment. The experiential Hands-on GMP Workshop will familiarise participants with working in an industrial environment where they can practice GMP related activities. The simulated environments for the training are either TRI T3 Cleanrooms Brisbane, Queensland, UTS Biologics Innovation Facility (BIF) Sydney, NSW and CBE Pure Solutions, Melbourne, Victoria. The facilities can accommodate 8 - 12 participants for the on-site training. These may also be conducted at the client premises by agreement.
Each module will an have assessment and at completion of the Essential GMP Uplift Program participants will receive a Certificate and a certified digital credential badge for students to use and record their education history in their professional history. As an outcome by the end of the training program, attendees will have broad understanding of GMP principles, quote specific examples, understand basic GMP application in case studies and have practical hands-on experience of base concepts to operate entry level tasks in a GMP environment.
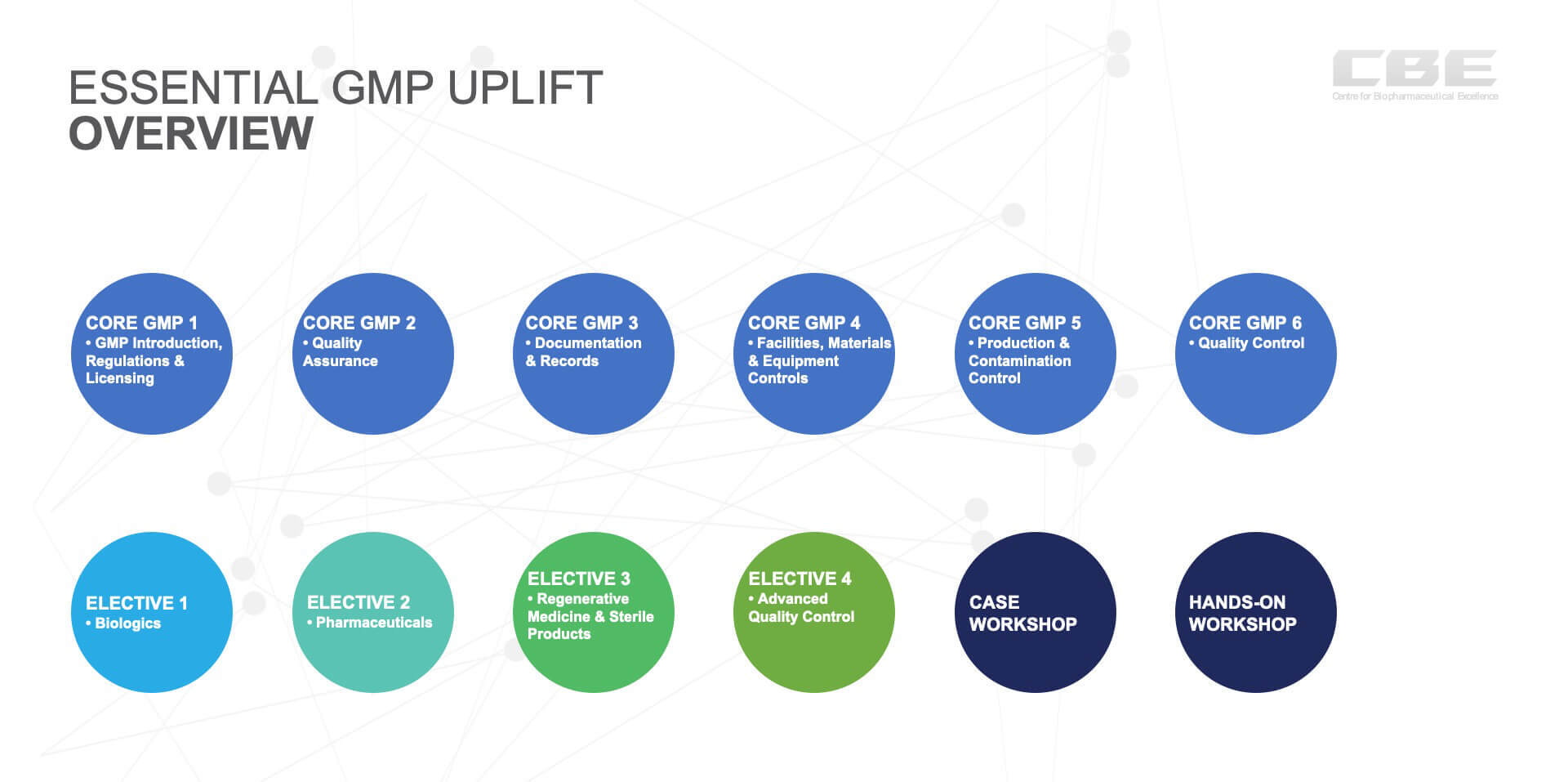
Essential GMP Program Outline
| Course | |
|---|---|
| CORE GMP 1 Introduction to GMPs, Regulations and Licensing |
|
| CORE GMP 2 Quality Assurance |
|
| CORE GMP 3 GMP Documentation and Records |
|
| CORE GMP 4 Facilities, Cleanrooms, Materials and Equipment Controls |
|
| CORE GMP 5 Production and Contamination Control |
|
| CORE GMP 6 Quality Control |
|
| Elective 1 Biologics |
|
| Elective 2 Pharmaceuticals |
|
| Elective 3 Regenerative Medicine & Sterile |
|
| Elective 4 Advanced Quality Control |
|
| Case Workshop |
|
| Hands-On Workshop |
|
The program has been designed to be agile and for flexibility, it can be run via online, webinars and virtual facilitation or “in-house” at company locations face-to face or a combination by arrangement with the client. Working with REDI and the industry around needs, CBE will offer companies the option depending on trainee numbers, external environment and feedback received.
Essential GMP Program Schedule
The published schedule for the 2025 Essential GMP Program.
2026 Schedule
Online Programs open - register today
Full Program Webinars Workshops on demand for industry cohorts - please contact us expression of interest
Email: gmpuplift@cbe-ap.com.au
Cohorts | Essentials Program Welcome and Information Session | Elective Q&A Sessions~ | Workshop Site | GMP Case Study Workshop* | GMP Hands-On Workshop* | GMP Hands-On Workshop^ | Status> |
| Industry Cohort | TBC | TBC | TBC | TBC | TBC | TBC | EOI Now |
~Webinar Sessions, one for each Elective, including Elective 01 Biologics, Elective 02 Pharmaceuticals, Elective 03 Regenerative Medicines & Sterile Products, Elective 04 Advanced Quality Control
*Availability subject to cohort numbers
^By arrangement >Graduation date is subject to completion of the 6 Core modules, 2 Elective modules and 2 Workshops

Click here to register for a place in the GMP Uplift Program
Places per cohort are limited, so register your interest today to secure a place, discuss company group registration or email gmpuplift@cbe-ap.com.au.
Essential GMP Program Testimonials
What our recently graduated participants are saying about CBE's Essential Programs , refer to their Testimonials:
“Everyone working or intending to work in a GMP environment should complete the program. It gives you the essential knowledge and the basis to carry out work in GMP correctly and with the right mindset.”
"A great introduction to GMP. The theory was very structured and clear to understand"
"Very thorough training and I think everybody has taken something away...I am very happy with what's been covered."
"Really enjoyed the case studies. They provide the opportunity to work through a real-life example and see how to apply GMP rules in the workplace. Its a great way to go from reading GMP rules to learning about the theory and how to apply it in the real world."
"It's great to be able to do this part [workshops] of the course face to face and have that hands-on experience that explains why we're doing what we are doing."


Case Studies
With participants from more than 85 companies having completed in the GMP Uplift Essentials programs so far, there are many examples where upskilling has been put into practice to help organisations to or set up to manufacture their medical products onshore.
- UNSW RNA Institute - GMP training uplifts RNA Institute, case study
- EnGenIc - From nanotech breakthroughs to GMP excellence: EnGeneIC’s quest to transform cancer treatment, case study
- World Mosquito Program - Advancing Global Health: The World Mosquito Program’s Journey with GMP Uplift, case study
- Vital Trace - Empowering Innovation in MedTech: How the GMP Uplift Essentials Program Supports VitalTrace’s Mission, case study
- BioCina - Embraces the GMP Uplift Essentials Program to Enhance Workforce Excellence, case study
Learning Management System (LMS)
GMP Uplift course material is available via the LMS, once your registration has been approved, students can access the LMS.
Click here to log into the GMP Uplift Learning Management System.
Advanced GMP Uplift
Training for those Experienced in GMP, up to Senior Level Managers
Current GMP requirements are increasingly demanding and complex and getting it right first time has significant impacts on quality, regulatory compliance and business efficiency. Modern managers need to understand and work across different disciplines as part of a cohesive team. The six short courses are based on consultation with industry and our consulting experience where we often see knowledge gaps and sub-optimal application of these disciplines. In particular, the application of risk management has significant leverage over the other five topics and will be a common theme throughout the series.
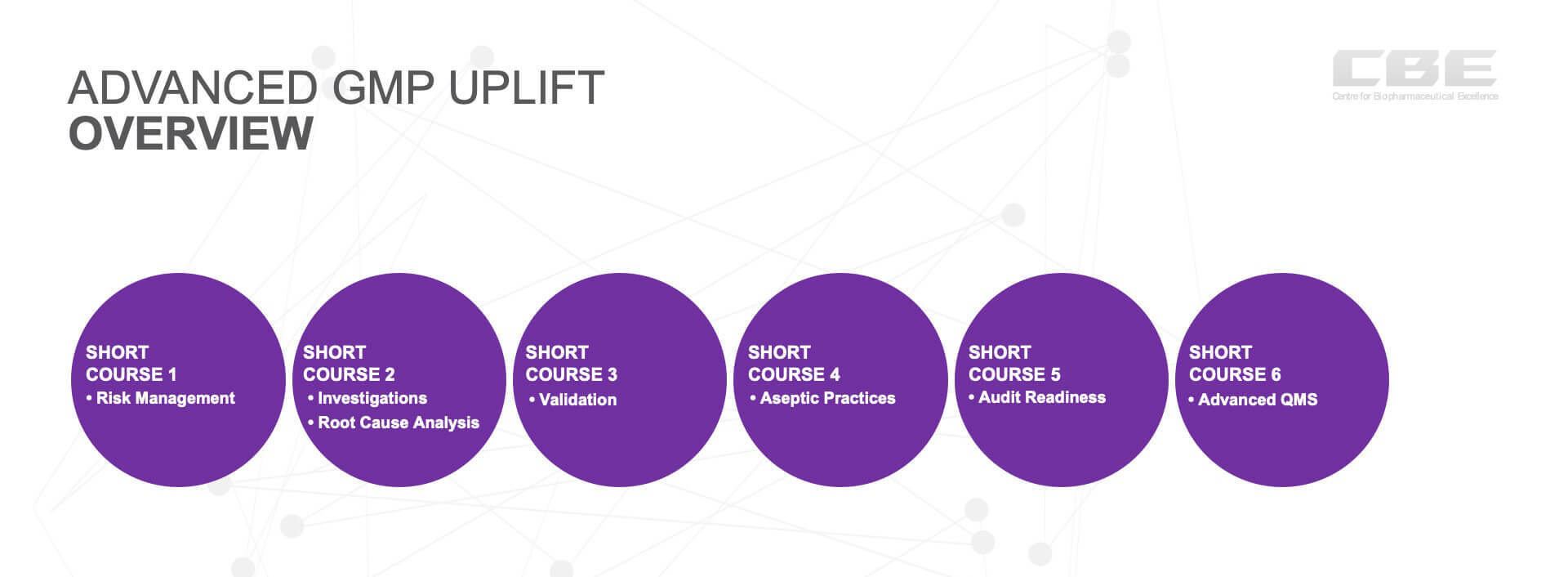
Advanced GMP Program Outline
The six courses are complementary and designed to super charge attendees for a more in depth understanding of critical areas that underpin GMP compliance in practice.
| Short Courses | |
|---|---|
| Risk Management | Risk is a foundational concept, that can be applied to all other areas of Quality Management. This course will cover the expectations of Quality Risk Management (QRM) in the MTP sector. Participants will learn to identify and apply the processes of QRM, the qualitative and quantitative tools to assess risk and classify risk into classes and different applications for reactive vs proactive situations. As well as how to organise, facilitate and participate in effective risk assessments and constructing risk management reports. |
| Investigations and Root Cause Analysis | Investigations and Root Cause Analysis (RCA) are vital tools for effective Corrective and Preventative Actions (CAPA) and Change management. This course covers problem investigations, RCA, CAPA and Change management, where participants will learn to identify key process steps for effective investigations, defining problems, investigation plans. Participants will learn the difference between symptoms and root causes, the application of QRM to determine actions, CAPA and continuous improvement via change management. |
| Validation | The language and terminology of qualification and validation can be very confusing, complex and easily misunderstood. This course explains correct terminology, introduces participants to science and risk concepts required, the industry requirements for qualification and validation, as well as how to determine a “validated state”. It will give participants practical knowledge and examples of how to leverage process understanding to deliver effective qualification and validation program, compliant with regulatory expectations. |
| Aseptic Practices | Aseptic processing and the GMP rules are particularly complex and require expert interpretation and application. This course will provide an overview of many of the GMP rules for aseptic processing and enable participants to interpret Annex 1 requirements, as well as manage the risks associated with aseptic processes. Participants will gain an appreciation of the expectations for compliance when manufacturing sterile products, including media fills, clean room classifications, particulate monitoring, microbial monitoring and interpretation of environmental monitoring trends. |
| Audit Readiness | Managing a regulatory inspection is critical to successfully maintaining GMP compliance or obtaining a GMP License. It is evident that a well prepared site has a much higher chance of success than one that is not prepared. Regulatory inspection can be stressful for all parties and everyone wants a balanced and fair outcome. This course explains what is expected from a regulatory inspection, how to apply the 4Ps approach and how to facilitate the right outcome from an inspection. Participants will learn how to be audit ready, prepare for an inspection, how interviews should be conducted and how to deal with any non-conformances. |
| Advanced QMS | Quality Management is considered the most important GMP system and as such the functioning of the Quality Management System (QMS) is central during regulatory inspections. A QMS consists of 10 fundamental elements and this course explains the process flows for most these elements. ICH Q10 now includes a continuous improvement requirement within the quality system and this is covered in the context of change management, quality metrics, auditing and management reviews. |
Delivered in a virtual, online environment, ideal for busy professionals. Theoretical concepts will be translated to the real world environment and be supported with Guest lectures, self directed learning, Q&A facilitated sessions and case work. A minimum of 12 months working in a GMP role or the GMP Essentials Program are pre-requisites for the course.
There is an online Welcome and Information Session, then each online Short Course will entail:
- Two to three hours of theory, practical application and guest lectures (self paced learning)
- One hour of facilitated Q&A Session with a CBE expert
- Case study work
- An online Assessment
Each Short Course will have an assessment and at completion of the Advanced GMP Uplift Program participants will receive a Certificate and Digital Credential.
Advanced GMP Program Schedule
The schedule for the 2026 Advanced GMP Program is now available.
2025-26 Schedule
| Cohort | Course Opens & Welcome Information Session | A01 Risk Management Webinar & Case Study | A02 Investigations & RCA Webinar & Case Study | A03 Validation Webinar & Case Study | A04 Aseptic Practices Webinar & Case Study | A05 Audit Readiness Webinar & Case Study | A06 Advanced QMS Webinar & Case Study | Status |
Q2 2025 | 25 Mar 2025 | 1 Apr 2025 | 8 Apr 2025 | 15 Apr 2025 | 29 Apr 2025 | 6 May 2025 | 13 May 2025 | Graduated |
Q3 2025 | 6 Aug 2025 | 13 Aug 2025 | 20 Aug 2025 | 27 Aug 2025 | 3 Sep 2025 | 10 Sep 2025 | 17 Sep 2025 | Graduating now |
| Q1/2 2026 | 16 Mar 2026 | 23 Mar 2026 | 30 Mar 2026 | 13 Apr 2026 | 20 Apr 2026 | 27 Apr 2026 | 4 May 2026 | Enrolments open |

Click here to register for a place in the GMP Uplift Advanced Program
Advanced GMP Program Testimonials
The participants from CBE's Advanced Programs have recently successfully completed the program and graduated, refer to their Testimonials about the program.
- “Even though I am not quite halfway through the course material so far, this is hands-down one of the most useful and practical GMP courses I’ve attended. The course material, the structure and format of each of the courses is spot on. I very much appreciated the approach that aimed at understanding and applying GMP rather than ‘you must do this because it will make the regulators happy’.” – Jacqueline
- “This month I completed the Advanced GMP Uplift program through CBE. This course was created to address key skill gaps in the Australian Biotech sector. Through this qualification, I greatly developed my understanding of the critical areas that underpin GMP compliance in practice. The program consisted of case studies, lectures and workshops on topics such as risk management, root cause analysis, audit readiness advanced quality management systems, aseptic processing and validation. An invaluable experience — I’m incredibly thankful to #MTPConnect, the guest lecturers, and the team at the Centre for Biopharmaceutical Excellence #CBE for providing this opportunity!” Henry
- “As a commercial leader exposed to the increasing government priorities in local ecosystem capabilities of Biopharmaceutical manufacturing, I have found the Advanced GMP manufacturing training by CBE under the MTP connect REDI program an incredibly useful investment of my time. It was practical and explained a complex area really simply which enabled by understanding despite my commercial background. I highly recommend it.” – Leah
- “This month I completed the Advanced GMP Uplift program through CBE. This course was created to address key skill gaps in the Australian #Biotech sector. Through this qualification, I greatly developed my understanding of the critical areas that underpin GMP compliance in practice. The program consisted of case studies, lectures and workshops on topics such as risk management, root cause analysis, audit readiness, advanced quality management systems, aseptic processing and validation. An invaluable experience — I’m incredibly thankful to MTPConnect, the guest lecturers, and the team at the Centre for Biopharmaceutical Excellence #CBE for providing this opportunity!”
- “I found the course very informative and relevant. I have started to apply my learning from the course in my current role. It has helped a lot to answer many questions I had about processes related to product development and scale-up to commercial manufacturing.”
- “The program was filled with useful tools which can be easily applied for GMP manufacturing processes. They are easily adapted to our company's situation and the presenter's explanation covered the 'why' as well as the 'how' which provides a great base to develop processes which are applicable to our situation.”
- “I have been able to contribute more effectively to risk assessments and practical solutions based on the knowledge I have gained in this course.” “As a direct result of the GMP Uplift Program, we have been able to standardize our company's approach to managing risk in a way that is clearly understood by all and is easily explained to customers and relevant authorities.”
- “I feel more confident in my existing knowledge but also in my approach to planning future work with this enhanced GMP knowledge.”
Learning Management System (LMS)
The GMP Uplift course material is available via the LMS, once your registration has been approved, students can access the LMS.
Click here to log into the GMP Uplift Learning Management System.
